
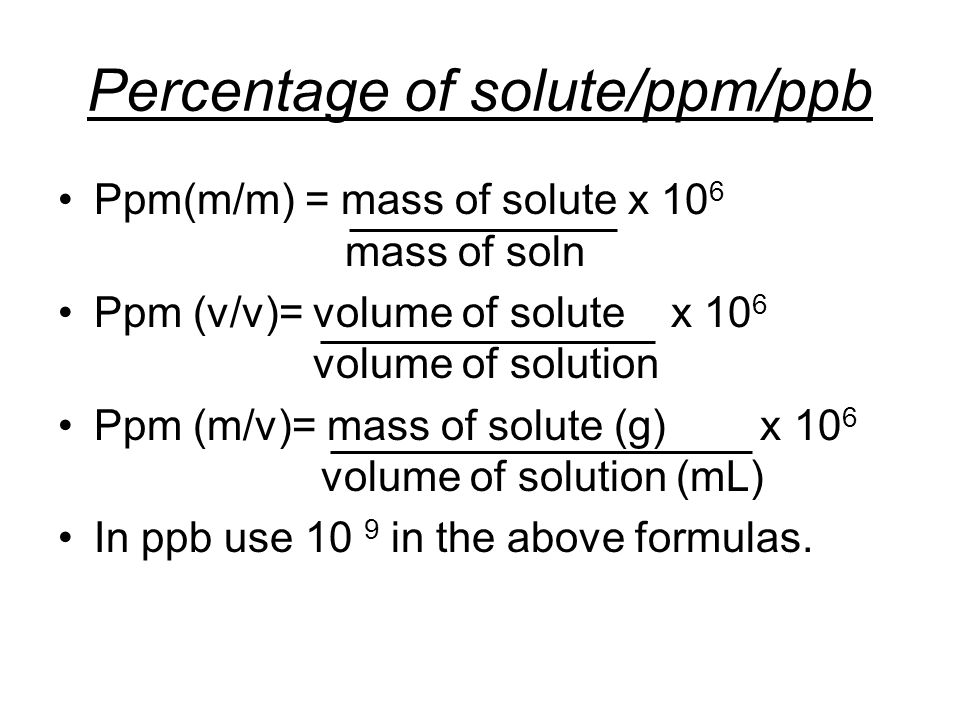
When ppm is used as a measure for the suspended particle concentration, it is therefore very important to specify if the concentrations are ppm BY VOLUME or ppm BY MASS, to facilitate comparisons with data where the concentrations are reported in µl/l or mg/l.ĭo you have a need for an instrument that can measure particle concentration? Have a look at our LISST-Portable|XR laser for laboratory use, our LISST-200X laser for field and submersible use, or our LISST-AOBS that measures concentration using acoustics. Given below is the representation of PPM in percentage. For example, a sample with a mass concentration of 100 mg/l will have a volume concentration of 38 µl/l. That is 1000 ml is one liter, so that 1 ppm 1 mg per liter mg/Liter. To convert from ppm by mass to ppm by volume, divide by the density of the particles.

For example, a sample with a volume concentration of 25 µl/l will have a mass concentration of 25*2.65 = 66 mg/l.
For mineral grains (clay, silt and sand sizes), this will typically be 2.65 g/cm3. To convert from ppm by volume to ppm by mass, multiply by the density of the particles. As difficult is it may seem on the surface, converting parts per million (ppm) to volume is actually very simple Think of 1 ppm as 1/1,000,000 which. However, if ppm is expressed as THE MASS of particles in a unit volume of water, then ppm BY MASS is equal to mg/l. Now we need to express the composition in grams and determine the number of moles of each element: Next we divide by the smallest number of moles to obtain the mole ratio which is also the atom. Since the sample contains C, H, and O, then the remaining. mass of solute (g) ppm × volume of solution. First we need to calculate the mass percent of Oxygen. If ppm is expressed as THE VOLUME of particles to a unit volume of water, then ppm BY VOLUME is equal to µl/l. Parts per million Calculations To calculate mass of solute: mass of solute (mg) ppm × volume of solution (L). But what parts? The amount of particles in a suspension can be expressed as the total volume OR total mass of particles in a unit volume of water AND THESE TWO NUMBERS WILL ONLY BE THE SAME IF THE DENSITY OF THE PARTICLES IS 1 g/cm3.


 0 kommentar(er)
0 kommentar(er)
